OVERVIEW
Trusted vessel sealing technology1 meets next-level performance.2,†,‡
The LigaSure™ XP Maryland jaw sealer/divider is advancing surgical energy to meet the evolving needs of surgeons and their patients.
With the LigaSure™ XP Maryland jaw device, you get all the advantages of the LigaSure™ technology you’ve come to love, plus additional next-level benefits:
- The confidence to reliably seal thick tissue3,4,§,||
- Precise sealing,5,¶ cutting, and dissection3,#,†† at the tip of longer Maryland jaws6,‡‡,§§
- Continuous 360° jaw rotation boosts procedural efficiency through undisrupted movement3,||,††
- Nano-coating performance consistent with legacy LigaSure™ devices,3,7,|| resulting in reduced tissue sticking compared to competing devices7,||||
Get ready to experience next-level performance, precision, and procedural efficiency2,†,‡,¶¶ with the LigaSure™ XP Maryland jaw device.
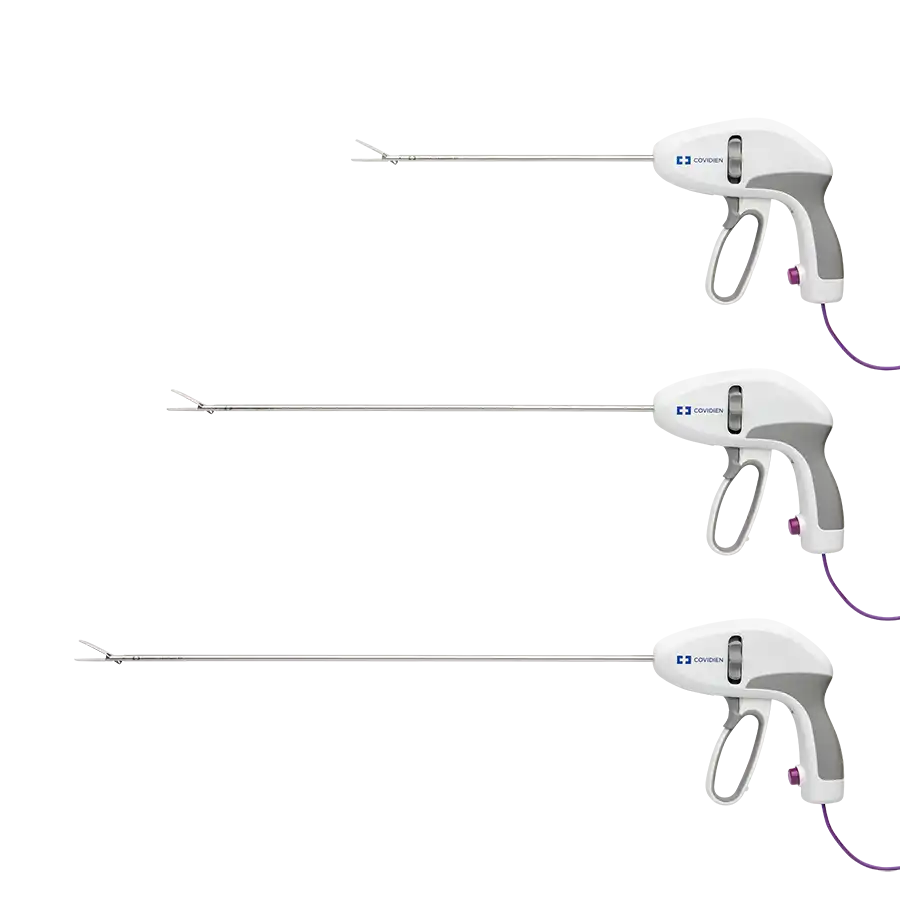
AN ELEVATED LEVEL OF DEVICE PRECISION1,†,‡
- Curved, tapered jaws enable fine dissection and skeletonization of vessels3,Ω
- Provides sealing5,§§ and more precise cutting3,ΩΩ at the tip of the jaws
- Jaw design makes it easier to access tissue planes or create windows3,‡‡,ΩΩ
NEXT-LEVEL PERFORMANCE, PRECISION, AND PROCEDURAL EFFICIENCY2,†,‡,¶¶
Take a deeper dive into the vessel sealing and dissection advantages of the LigaSure™ XP Maryland jaw device.

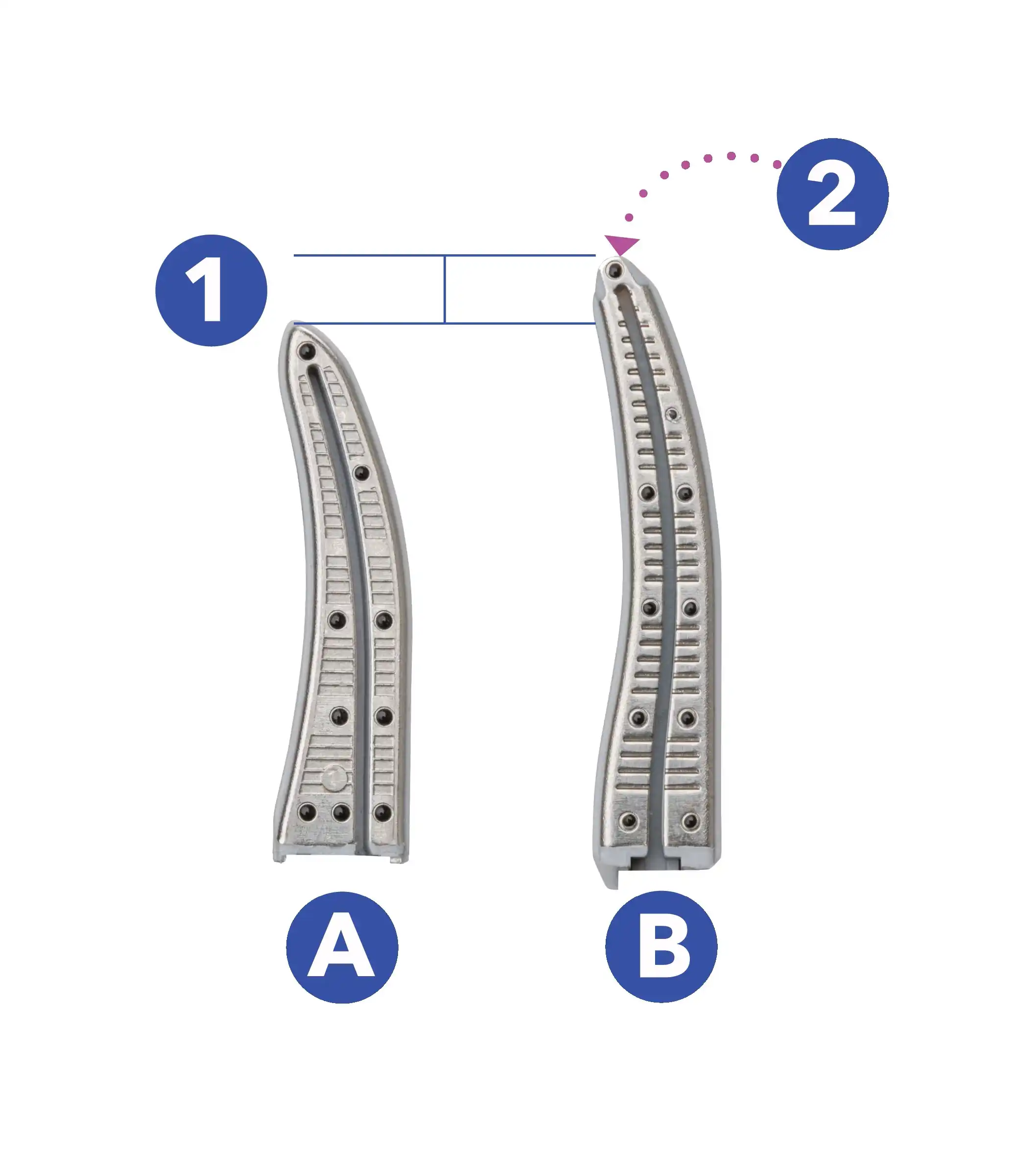
SEE THE DIFFERENCE OF A LONGER JAW
1. 3.1 mm longer6,§§
2. Vessel sealing5,¶ and cutting3,#,†† to the tips of the jaws
A. LigaSure™ Maryland jaw
B. LigaSure™ XP Maryland jaw
VESSEL SEALING AND DISSECTION THAT OUTPERFORMS THE COMPETITION
Compared to the Ethicon Enseal™* X1 device, the LigaSure™ XP Maryland jaw device provides:
- Greater average burst pressure on isolated large vessels (5.1–7.0 mm)8,||||
- Higher average burst pressure in thick tissue4,§,||||
- A finer jaw6,## that grasps6,††† and transects6,‡‡‡ more tissue per bite while cutting closer to the device tip6,§§§
- † The LigaSure™ XP Maryland jaw device is indicated for use in general surgery and such surgical specialties as colorectal, bariatric, urologic, vascular, thoracic, and gynecologic.
- ‡ Compared to their current preferred device; 21 of 23 surgeons agreed during clinical procedures.
- § Thick tissue is defined as nondissected vascular tissue or fatty tissue.
- || 29 out of 29 surgeons agree.
- ¶ 30 out of 30 surgeons agree.
- # 24 out of 29 surgeons agree.
- †† Compared to legacy LigaSure™ devices.
- ‡‡ LigaSure™ XP Maryland seal plate length (22.555 mm) vs. LigaSure™ Maryland seal plate length (19.406 mm) as measured from seal plate tip to device tissue stop.
- §§ LigaSure™ XP Maryland cut length (21.844 mm) vs. LigaSure™ Maryland cut length (18.034 mm).
- |||| Bench tissue may not be indicative of clinical performance.
- ¶¶ Compared to their current preferred device; 20 of 23 surgeons agreed during clinical procedures.
- ##LigaSure™ XP Maryland jaw width by height (1.47 mm by 2.13 mm) vs. Ethicon Enseal™* X1 width by height (1.57 mm by 2.90 mm).
- †††LigaSure™ XP Maryland jaw length & aperture (23.368 mm & 14.437 mm) vs. Ethicon Enseal™* X1 jaw length & aperture (22.682 mm & 13.462 mm) as measured from device tip to tissue stop.
- ‡‡‡LigaSure™ XP Maryland cut length (21.844 mm) vs. Ethicon Enseal™* X1 cut length (21.133 mm).
- §§§LigaSure™ XP Maryland uncut seal length on seal plate (1.143 mm) vs. Ethicon Enseal™* X1 uncut seal length on seal plate (1.727 mm).
- 1. Based on COGNOS and historical sales data, FY01–FY20. October 2019.
- 2. Based on internal report #RE00457416 Rev A, Product introduction report: LigaSure™ XP Maryland jaw sealer/divider. May 2023.
- 3. Based on internal report #RE00376094 Rev A, LigaSure™ XP Maryland jaw sealer/divider surgeon validation marketing report. Dec. 7–9 and 14–16, 2021.
- 4. Based on internal report #RE00442444 Rev A, Comparison of the renal artery bench bundle burst pressure performance with the LigaSure™ XP Maryland jaw sealer/divider, Voyant™* Maryland Fusion, Enseal™* X1 curved jaw, and LigaSure™ LF19XX devices. Jan. 23, 2023.
- 5. Based on internal report #RE00372649 Rev A, Archer surgeon summative evaluation report. March 22, 2022.
- 6. Based on internal test report #RE00455509 Rev B, Competitive device analysis — LigaSure™ XP Maryland jaw sealer/divider.
- 7. Based on internal report #RE00380835 Rev B, Sticking evaluation with the LigaSure™ XP Maryland jaw sealer/divider, Aesculap Caiman™* 5 Maryland, Voyant™* Maryland Fusion, Ethicon Enseal™* X1 curved jaw, LigaSure™ LF18XX blunt tip, and LF19XX Maryland devices.
- 8. Based on internal report #RE00380822 Rev A, Comparison of the renal artery seal burst pressure performance with the LigaSure™ XP Maryland jaw sealer/divider, Caiman™* 5 Maryland, Voyant™* Maryland Fusion, Enseal™* X1 curved jaw, Sonicision™ 7 curved jaw, Harmonic™* 1100, LigaSure™ LF18XX, and LF19XX devices.
 Ligasure™ XP_Brochure_EN
Ligasure™ XP_Brochure_EN

 Microsystem
Microsystem Endoscopysystem
Endoscopysystem Energysystem
Energysystem +86-21-54286005
+86-21-54286005
 info@tenmed.net
info@tenmed.net
 Room 602, Building 1, No. 111 Luxiang Road (Greenland Park Plaza), Baoshan District, Shanghai, China
Room 602, Building 1, No. 111 Luxiang Road (Greenland Park Plaza), Baoshan District, Shanghai, China

 Ligasure™ XP_Brochure_EN
Ligasure™ XP_Brochure_EN




